
What is corrosion? Why does it occur?
What is corrosion?
The degradation of metals over time due to interactions with their environment
– by Jeroen Fijan, 16 June 2016
As Heinen & Hopman products and systems are deployed worldwide and in many different environments, the materials we use are exposed to all kinds of corrosion. This is why corrosion protection is a must.
Heinen & Hopman invests enormous time and energy in selecting the right materials and taking preventive measures against corrosion. This series of blogs will highlight the vital need for knowledge about corrosion and preventive measures. We start here by focusing on the causes of corrosion and the conditions under which it occurs.
What is corrosion?
While corrosion is often associated with rust, this is actually just a side product. In general corrosion is the degradation of metals over time due to interactions with their environment.
Why does corrosion occur?
In contrast to when extracted, once it is used an unprotected (semi-)finished metal is in its pure form. Almost all metals are mined in a compound/ore form (e.g., sulphide or oxide). Simply put: the metal corrodes because it is not in its natural (ore) state and is trying to return to that state.
When does corrosion occur?
Metal corrosion is generally an electrochemical process. This means that it requires three preconditions to start the corrosion process, namely:
- There is potential;
- There is an electrolyte which is in contact with the metal;
- There is a closed circuit.
Explanation of the preconditions
The ‘potential’ is the voltage difference between the two metals in the electrochemical cell, also known as the anode and cathode. ‘Electrolyte’ is the connecting medium between the anode and cathode which conducts the current. In practice the electrolyte is often water and the corrosion occurs on the anode. The final precondition is that the circuit is closed, which means that the two metals are touching. There will be no active corrosion unless all of these conditions are met.
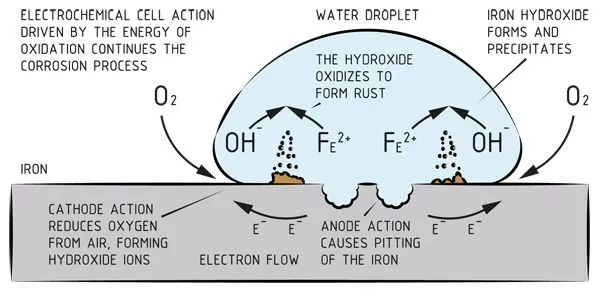
Corrosion is therefore not a property that is inextricably linked to a material; it can better be described as an interaction between materials and their environment.
Source: Budinski, K. G., & Budinski, M. K. (2002). Corrosie. In M. Kooijman & M. Pallada (Eds.), Materiaalkunde voor technici (pp. 479-517). Den Haag, the Netherlands: Academic Service.
Jeroen Fijan | R&D Manager
Jeroen Fijan has been working at Heinen & Hopman since 2001. He started as a draughtsman and, over the years, worked his way up to the top of the R&D division. Sustainability is a top priority in the quest to improve H&H’s products and processes.