
Water, the ultimate energy carrier
Water, the ultimate energy carrier
09-12-21
Ancient Greek and Roman engineers already used water to cool down their houses on summer days. And it worked. Today we still use water as a medium to heat or cool our homes. Why water has been the preferred energy carrier for over 2000 years is the subject of this blog.
Specific heat capacity
Why is water such a good medium for cooling? Even on a hot summer day, seawater at the beach or in a lake brings refreshment and is much cooler than the surroundings. You can imagine that fresh flowing water is cool, but the reason is due to a physical property called the specific heat capacity. Let’s look at some important physical properties.
|
Water 20°C | |
|
Specific heat |
4.2 kJ/kg.K |
|
Specific mass |
1,000 kg/m³ |
While the specific mass tells us something about the density of water – namely 1,000 kg for one cubic metre of water – the specific heat tells us how much energy water contains.
The amount of energy needed to heat up an amount of 1kg water and let it rise 1 Kelvin is 4.2 kJ. This is best shown by an example.

This aquarium is filled with 2 cubic metres of water at a temperature of 20°C.
How much heat (thermal energy) do we need to add to this aquarium to raise the temperature by 1 Kelvin to 21°C?
If 1m³ of water weighs 1,000 kg, 2 m³ of water weighs 2,000 kg.
The specific heat of water is around 4.2 kJ/kg.K.
To raise the temperature of 2,000 kg of water, we therefore need 2,000 x 4.2 kJ of energy. That’s 8,400 kJ of heat required to raise 1°C – a massive amount of energy and proof that it is not easy to increase the temperature of water.
Therefore, water stays so cool and the ancient Greeks used it to cool their cities.
Water vs air
The specific heat capacity of air is significantly lower than that of water. On top of that, it obviously weighs less than water.
|
Air 20°C | |
|
Specific heat |
1.0 kJ/kg.K |
|
Specific mass |
1.2 kg/m³ |
Let’s take the same conditions from our previous example and reflect it to air.
Two cubic metres of air only weigh 2.3kg. The specific heat capacity is 1 kJ/kg.K
To raise the temperature of 2.3 kg air we need 2.3 kg x 1 kJ/kg of energy. That is 2.3 kJ of thermal energy for an increase of 1°C.
This is the reason why a relatively small water-fed cooling coil has enough capacity to cool down an entire room. It also works the other way around. When heated, it can store a high amount of thermal energy that is easily released to its surroundings, which is the case with central heating.
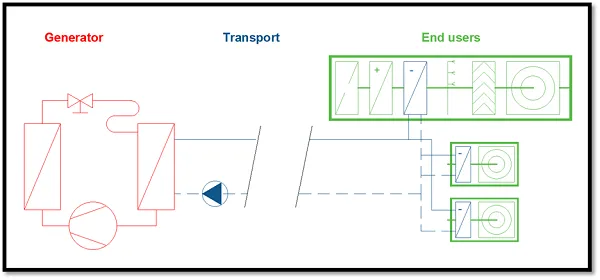
The systems
Heat and cold are generated by the boiler and cooling machine/chiller respectively. These machines heat up or cool the water in the system.
Water is used as energy carrier. It carries either the cold energy or heat energy, transporting it to the specific end users such as air handling units, local heaters or fan-coil units. They then cool or heat the room.
In summary:
- Generators: chiller or boiler;
- Transport system: system of pipes transporting the water;
- End users: water-fed heater, coil inside air handling unit or fan-coil unit.
Latent heat capacity
Water is such a good energy carrier because of its high specific heat capacity. It needs a lot of (thermal) energy before it raises in temperature. The highest heat capacities are achieved when the medium changes in phase. Like, when water is boiling: no temperature rise can occur, and all the thermal energy is used to change the liquid into vapour.
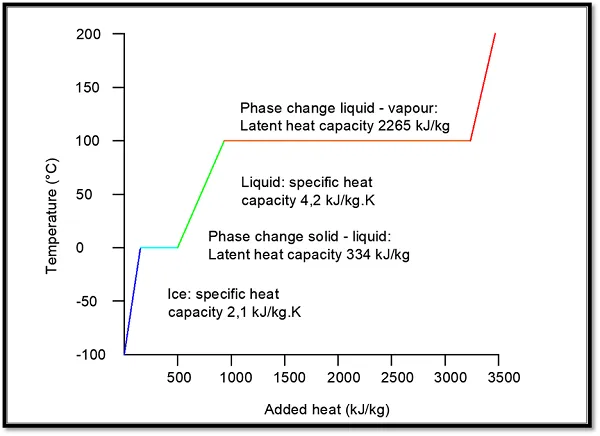
Turning water from liquid into vapour at an atmospheric pressure costs 2,265 kJ/kg. This so-called latent heat capacity is the driving force behind a cooling machine, but it can also be used for the storage of heat and cold.
Innovative ways for energy storage
Although water is a perfect medium for active cooling/ heating that has been used for over 2000 years; there are more innovative ways to store thermal energy. One of them is phase changing material which, as the name suggests, make use of latent heat capacity when changing in phase.
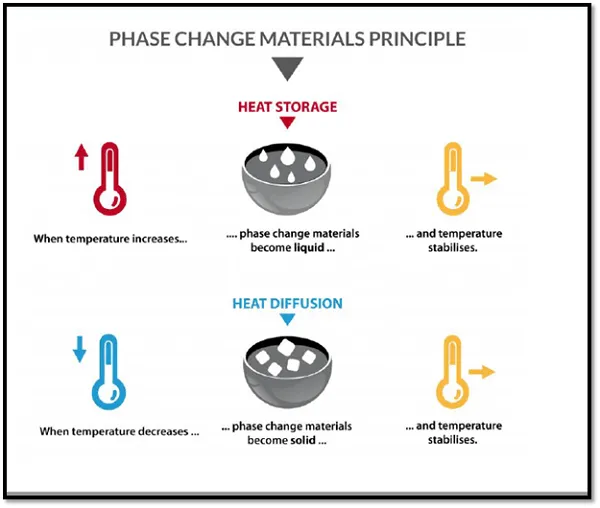
An example of PCM’s (phase changing materials) are solid plates that contain a substance with a melting point around room temperature. When temperature rises, the thermal energy is used to melt the substance. While this cost a lot of thermal energy, it prevents the room from heating up, but stores it into the plate. The heat is absorbed into the PCM.
As the temperature goes down, the liquid turns into a solid, releasing the stored thermal energy to its environment. For more information about PCM’s read this article.
Phase changing LNG
Another sustainable way to make use of latent energy stored in a medium is done with LNG cooling. For environmental purposes, more ships use Liquid Natural Gas (LNG) as a fuel. To make it easier to transport the volume is reduced by cooling the LNG down (-162°C) so it becomes liquid. When used as a fuel, it needs to change phase, from liquid to gas. During this process, the cold energy or thermal energy, stored inside the liquified LNG is used for cooling purposes.
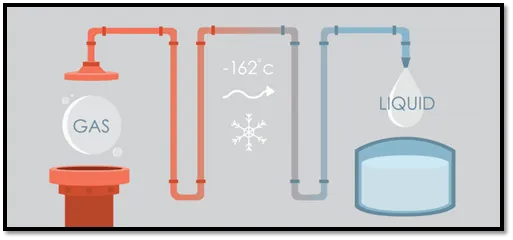
Of course, water is used to transport the thermal energy to the end users. More on LNG cooling you will find in this article.
Conclusion
The thermodynamic properties of water make it an excellent energy carrier for both cooling and heating purposes. It is a dense medium that can contain and absorb a great deal of thermal energy. In addition, it has a low viscosity which makes it easy to pump, it is available everywhere and cheap.
When a medium changes in phase, from solid to liquid, or liquid to gas, a lot of thermal energy is needed. This property can easily be used to store thermal energy and use it for sustainable purposes.
Want to stay up to date with the latest blogs, news and information about maritime HVAC? Sign up for our newsletter, or regularly check heinenhopman.com.